En-en adult card 23 hindered calcification process: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
m (Minor corrections) |
||
| Line 4: | Line 4: | ||
|title=Hindered Calcification Process | |title=Hindered Calcification Process | ||
}} | }} | ||
== Explanation == | == Explanation == | ||
The formation of limestone (calcification) follows the chemical reaction Ca<sup>++</sup> + 2HCO<sub>3</sub><sup>-</sup> ⇔ CaCO<sub>3</sub> + H<sub>2</sub>O + CO<sub>2</sub> | The formation of limestone (calcification) follows the chemical reaction Ca<sup>++</sup> + 2HCO<sub>3</sub><sup>-</sup> ⇔ CaCO<sub>3</sub> + H<sub>2</sub>O + CO<sub>2</sub> | ||
| Line 18: | Line 12: | ||
The addition of an acid shifts the equilibrium towards the left of the equation. In other words, if the pH drops, there are fewer bicarbonate ions, making it more difficult for organisms to build their shells. | The addition of an acid shifts the equilibrium towards the left of the equation. In other words, if the pH drops, there are fewer bicarbonate ions, making it more difficult for organisms to build their shells. | ||
== Correction== | |||
===Causes=== | |||
* [[En-en_adult_card_24_ocean_acidification|Ocean Acidification]] | |||
=== Consequences === | |||
* [[En-en_adult_card_29_pteropods_and_coccolithophores|Pteropods and Coccolithophores]] | |||
[[fr:Fr-fr_adulte_carte_23_problèmes_de_calcification]] | [[fr:Fr-fr_adulte_carte_23_problèmes_de_calcification]] | ||
Revision as of 16:15, 5 April 2021
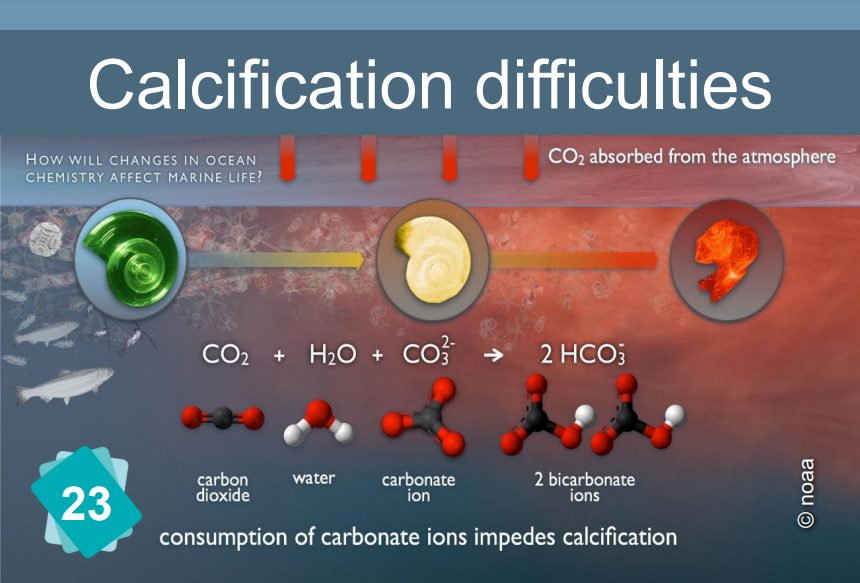
Card #23: Hindered Calcification Process
| Causes | Consequences | |
|
When the pH drops, the formation of calcium carbonate (and more specifically, of calcified shells) becomes more difficult.
Explanation
The formation of limestone (calcification) follows the chemical reaction Ca++ + 2HCO3- ⇔ CaCO3 + H2O + CO2
It requires the presence of bicarbonate ions (HCO3-). However, the quantity of these ions in water depends on the pH: in water, carbon dioxide, carbonic acid, bicarbonate ions and carbonates ion are in equilibrium, depending on the pH :
CO2 + H2O ⇔ H2CO3 ⇔ H+ + HCO3- ⇔ 2 H+ + CO32-.
The addition of an acid shifts the equilibrium towards the left of the equation. In other words, if the pH drops, there are fewer bicarbonate ions, making it more difficult for organisms to build their shells.